GPAINNOVA’s RESPIRA emergency ventilation device receives the approval of the AEMPS to extend its clinical trial to 45 hospitals of the Sole ICU of Catalonia
- Agencia Española del Medicamento y Productos Sanitarios (AEMPS) authorized yesterday the extension of the use of the RESPIRA device to 45 hospitals that conform the Sole ICU in Catalonia.
- GPAINNOVA has started the manufacturing process of RESPIRA device and is able to supply more than 1.000 devices weekly to hospitals around the world.
Multinational company GPAINNOVA can now extend clinical trials to 45 hospitals that conform the Sole ICU of Catalonia, after receiving authorization from Agencia Española del Medicamento y Productos Sanitarios (AEMPS). The first clinical trial with patients affected with Covid-19 that is being carried out at Hospital Clínic is giving excellent results. From now on, the use of RESPIRA device can be extended to intensive care units of 45 hospitals in Catalonia that conform the named Sole ICU.
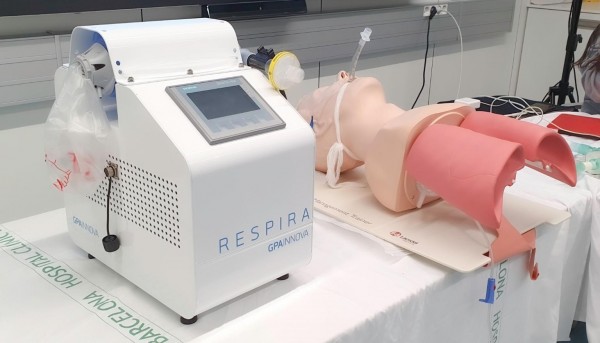
GPAINNOVA has already begun to produce these devices in order to respond immediately to the demand in hospitals. GPAINNOVA is already producing RESPIRA device, which can reach a production of 300 units per day. RESPIRA device has the support of specialized technologists as its main partner SIEMENS and also SMC, TEG and MAM to carry it out.

RESPIRA project has been developed under the supervision of Institut Català de la Salut (ICS) of catalonian Government with the participation of Hospital Clínic of Barcelona and Centre de Medicina Comparativa i Bioimatge del Institut de Recerca Germans Trias i Pujol – Can Ruti in Badalona, also with healthcare workers from Hospital de Sant Joan de Déu of Barcelona.
It has also had the support of Secretaría General de Industria y Pyme del Ministerio de Industria, Comercio y Turismo. RESPIRA project has been carried out following the guidelines of Agencia Española del Medicamento y Productos Sanitarios of the Ministry of Health.
PRESS CONTACTS
Press GPAINNOVA:
Betona Comín
Tel. 607 29 11 05
E-Mail: [email protected]
Press RESPIRA Project:
Jordi Miras Llopart
Tel. 678 43 98 39
E-Mail: [email protected]
Communication material link: https://www.dropbox.com/sh/dqvowhmtw3g71v3/AABnMudJBmOvQmGx6neTYUI2a?dl=0
Media Contact
Company Name: GPAInnova
Contact Person: Media Relations
Email: Send Email
Phone: (+34) 93 125 65 36
Country: Spain
Website: https://www.respiradevice.com/